2023-11-08
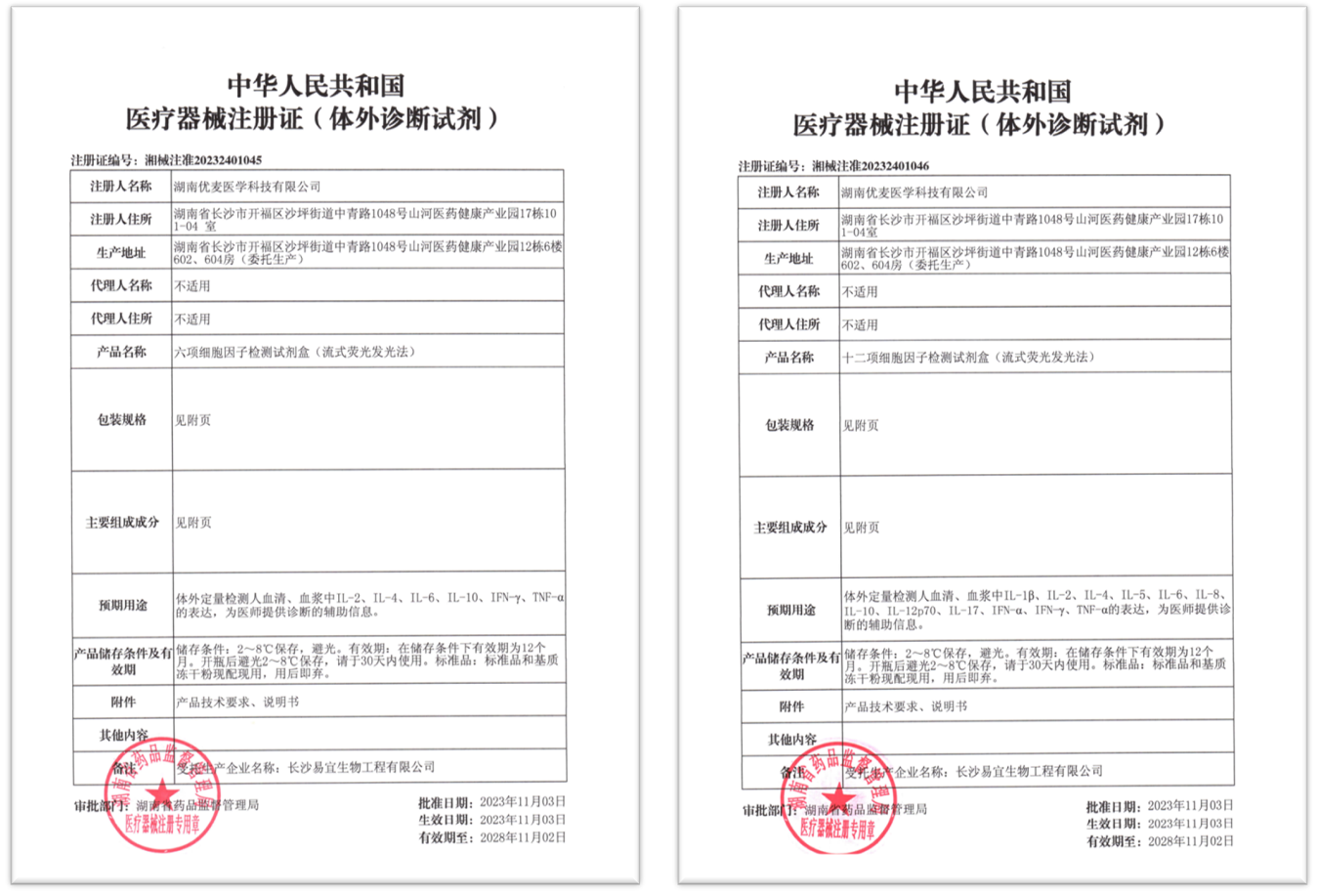
On November 3rd, Hunan Uni-medica (the subsidiary corporation of Shenzhen Uni-medica Technology Co., Ltd.) received the Class II Registration Certificate of Six Cytokine Detection Kits (Flow Fluorescence Luminescence Method) and Twelve Cytokine Detection Kits (Flow Fluorescence Luminescence Method) from the FDA Bureau, which marks a great achievement of Uni-medica in the field of flow cytometry.
Multiple cytokine detection, the new item is based on a flow cytometer platform to detect leukocytes, interferon and tumor necrosis factor, which is utilized to estimate various infections and immune status of the body. In clinical, it is utilized to conduct auxiliary diagnosis of infectious diseases, autoimmune diseases, and tumor status, etc., and to conduct illness assessment and prognosis judgment of diseases. With the widespread development and application of the project, it has been unanimously recognized by more and more clinical workers. More than ten guidelines have made standardized suggestions on implementing the project, which has become new index in clinical infections and immune testing.
This project is suitable for the departments that required for Infection and immune status assessment, and a number of guidelines recommend implementing this project in Intensive Care Department, Respiratory Department, Hematology Department, Infection Department, Oncology Department and other departments.