2022-03-03

The company takes the technical innovation of molecular detection methods as the core driving force, and focuses on the R&D and industrialization of molecular diagnostic products aiming at prevention and control of infectious diseases and genetic diseases. Uni-Medica Laboratory provides total solution of accurate diagnosis of infectious diseases and genetic diseases based on multi-omics technologies, including multiplex PCR, NGS technology and flow cytometry.

Uni-Medica Laboratory will rely on RT-PCR, High-Throughput sequencing and other technical platforms to provide COVID-19 Nucleic Acid Detection, Newborn Screening of Inherited Metabolic Diseases by NGS, and Targeted Sequencing (tNGS) for pathogenic microorganisms.
1 Novel Coronavirus Nucleic Acid Detection
Since the outbreak of COVID-19, shenzhen uni-medica has helped with the epidemic prevention and control in many places. During this period, it has accumulated rich experience in Novel Coronavirus Nucleic Acid Detection, and established an effective Novel Coronavirus Nucleic Acid Detection system. The establishment of the laboratory marks the coming of a new chapter of the uni-medica inspection work.

2. Newborn Screening of Inherited Metabolic Diseases by NGS
“Badyhealth” newborn screening of inherited metabolic diseases by NGS, using Uni-medica “All-in-One” mutiplex PCR amplicon library preparation technology and NGS technology, to detect 130 inherited diseases.

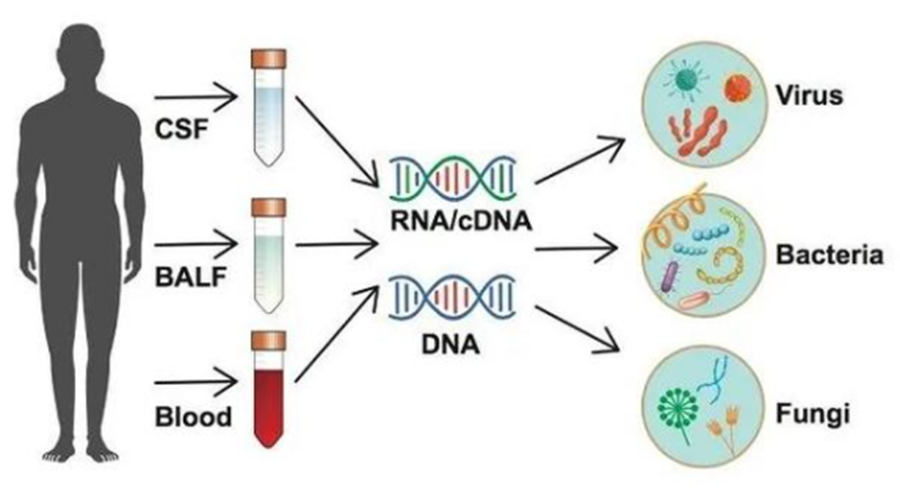
3. Pathogen Microbial Targeted Sequencing (tNGS)
Targeted sequencing of pathogenic microorganisms can provide a fast and accurate detection basis for the simultaneous detection of a large number of pathogenic microorganisms.