Multiplex Real time PCR Kit for HPV and 16/18 Typing
Multiplex Real time PCR Kit for 23 HPVs Genotyping
Multiplex Real time PCR Kit for 28 HPVs Genotyping
Multiplex Real time PCR Kit for HPVs E6/E7 mRNA Genotyping
Multiplex Real time PCR Kit for Mycobacterium tuberculosis and Non-Tuberculous Mycobacteria
Real time PCR Kit for Mycobacterium Tuberculosis RpoB Gene and Mutation
Multiplex Real time PCR Kit for Viral Diarrhea
Real time PCR Kit for Plasmodium
Real time PCR Kit for Dengue Virus
Multiplex Real time PCR Kit for ZIKV/CHIKV/DENV
Multiplex Real time PCR Kit for SARS-CoV-2 Influenza A Influenza B RSV
Rapid Real time PCR Kit for Monkeypox Virus
Multiplex Real time PCR Kit for NG/UU/MG/UP/ MH/TV
Real Time PCR Kit for Respiratory Pathogens
Total Solution of Novel Coronavirus 2019-nCoV Nucleic Acid Detection
2022-01-29
The Omicron variant, which was designated a “variant of concern” by the World Health Organization (WHO), has more than 30 mutations in the spike protein alone. The WHO has reported that preliminary evidence suggests an increased risk of transmission compared to other variants of concern.
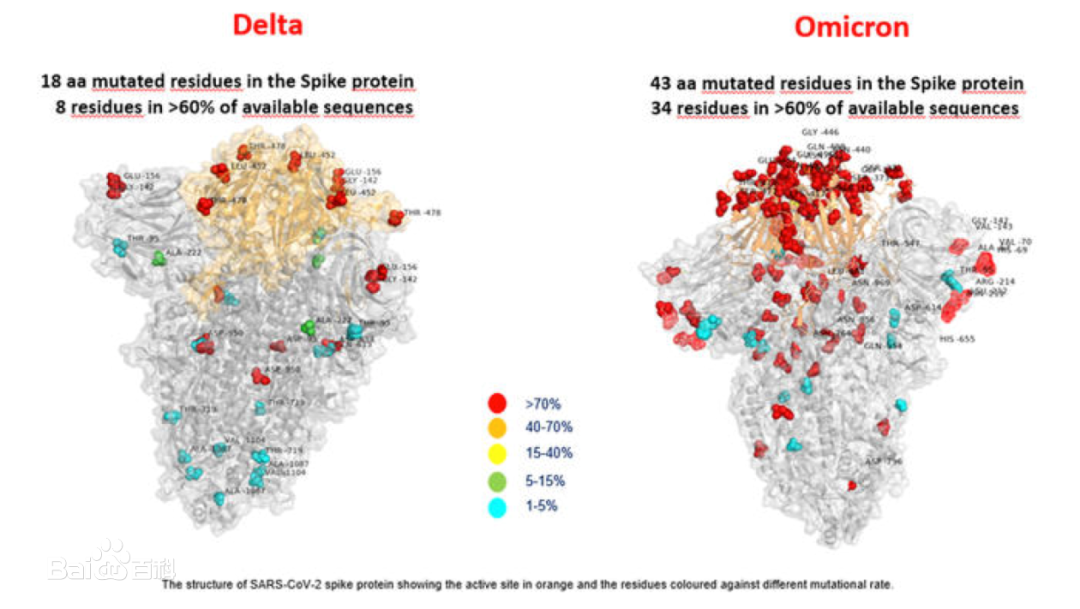
Since the report of the mutant strain, Uni-medica has paid close attention to its mutation and quickly started technical research. According to GISAID data, more than 95% of Omicron mutants have s gene hv69-70 del. At the same time, WHO, CDC in the United States and CDC in Europe and other authoritative institutions have issued documents suggesting that the hv69-70 del locus of S gene can be used as the PCR screening site of Omicron. Uni-medica has successfully developed the screening kit for Omicron mutant through a large number of biological information analysis and experimental verification. Recently,the kit has obtained CE certified. We also provide freeze-dried detection kit, making convenient for its transportation under room temperature.


