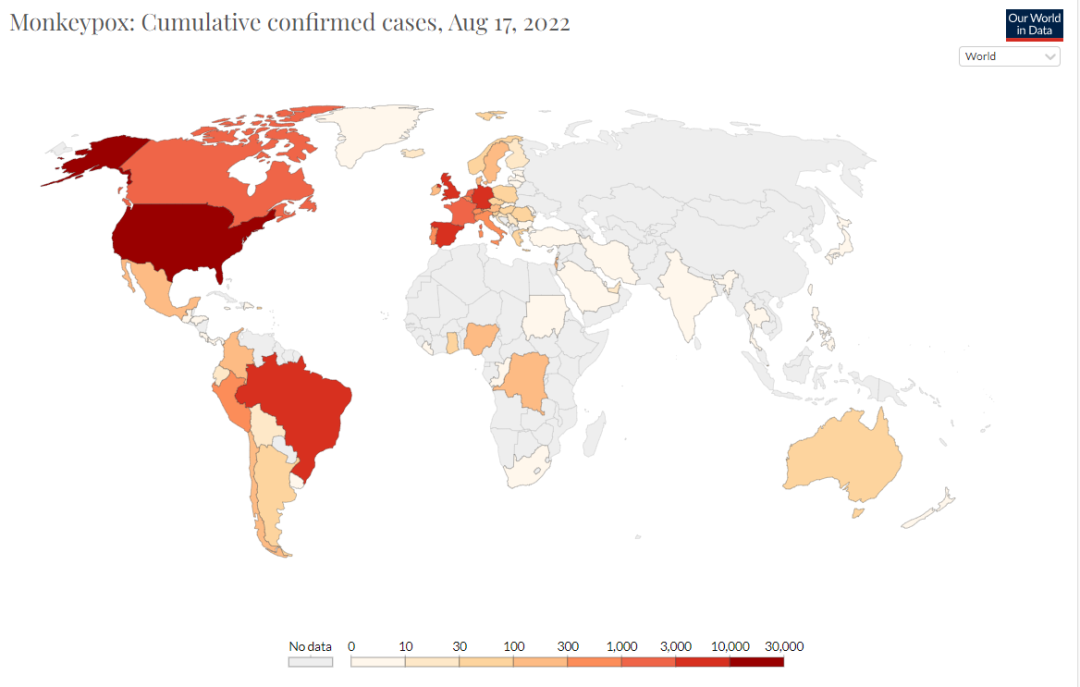
More than 35,000 cases of monkeypox, including 12 deaths, have been reported in 92 countries and territories, according to the World Health Organization.
WHO Director-General Tedros Adhanom Ghebreyesus reported the situation at a regular press briefing in Geneva, Switzerland. He said there were nearly 7,500 new cases of monkeypox last week, a 20 percent increase from the previous week, with the vast majority coming from Europe and the Americas.
Uni-medica Monkeypox Virus Test series is registered with the MHRA in the United Kingdom
Recently, Joint medical technology co., LTD. Research and development of the "fast monkeypox virus nucleic acid detection kit (fluorescence PCR method)", monkeypox virus nucleic acid detection and the central African/west branch points detection kit (fluorescence PCR method), monkeypox virus antigen detection kit (colloidal gold), monkeypox virus antibody detection kit (colloidal gold) four product for UK MHRA registration at the same time , the product can be sold in the UK countries and countries that recognise the UK MHRA registration.
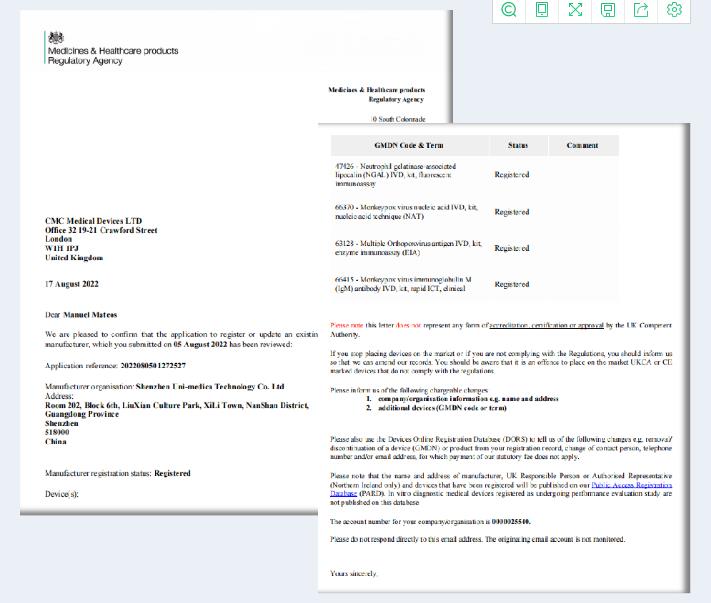
The MHRA stands for the Medicines and Healthcare Products Regulatory Agency. The MHRA is an executive government agency under the UK Department of Health, which ensures that medicines and medical devices are safe and effective. After Brexit, the UK will no longer recognize the EU CE certification according to the Brexit agreement. For medical devices, the CE certification can continue to be used in the UK until June 30, 2023, but the enterprises that need to hold the CE certification have a British person in charge in the UK (similar to the EU authorized representative), and the British person in charge of MHRA registration. To enter the UK GB market (England, Wales and Scotland). From 1 July 2023, CE certification will no longer be recognised and UKCA certification will be required.
Uni-medica monkeypox virus nucleic acid detection solution
The nucleic acid detection product of monkeypox virus developed by Uni-medica has obtained European CE certification and British MHRA certification. In the rapid PCR amplification mode, the detection result can be obtained in only 30 minutes, with short detection time and high sensitivity.
Joint medicine based on accumulated in infectious disease pathogens molecular detection technology for many years, in addition to the launch of monkeypox virus detection series products, also has a variety of pathogenic microorganisms of rapid detection kit, including respiratory, intestinal, entomophilous, genital tract, etc., and multiple kit have been granted NMPA, CE certification, used for rapid identification of pathogenic microorganisms.

.png)