2021-12-28
The B.1.1.529 variant was first reported to WHO from South Africa on 24 November 2021.
As of 22 December 2021, the Omicron variant had been identified in 110 countries across all six WHO Regions.

The Omicron variant, which was designated a “variant of concern” by the World Health Organization (WHO), has more than 30 mutations in the spike protein alone. The WHO has reported that preliminary evidence suggests an increased risk of transmission compared to other variants of concern.
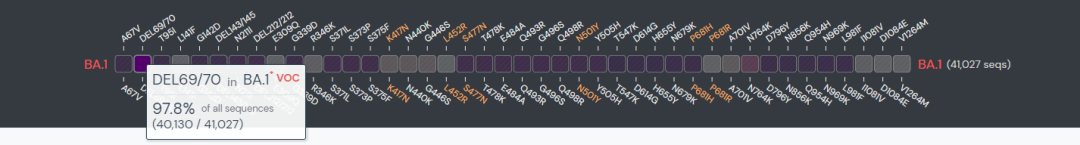
Since the report of the mutant strain, Uni-medica has paid close attention to its mutation and quickly started technical research. According to GISAID data, more than 95% of Omicron mutants have s gene hv69-70 del. At the same time, WHO, CDC in the United States and CDC in Europe and other authoritative institutions have issued documents suggesting that the hv69-70 del locus of S gene can be used as the PCR screening site of Omicron. Uni-medica has successfully developed the screening kit for Omicron mutant through a large number of biological information analysis and experimental verification.
The kit is used to detect the HV69-70 del locus of S gene. It also detects the COVID-19 O gene and N gene to meet the sensitivity and accuracy of the New Coronavirus detection. At the same time, one tube can realize the qualitative detection of New Coronavirus and the preliminary screening of Omicron mutant strain.
We, Shenzhen Uni-medica Technology Co., Ltd, Address: Room202, Block 6th, LiuXian Culture Park, XiLi Town, Nanshan District, Shenzhen, Guangdong Province, 51805S, P. R. China hereby declare that Uni-medica Real-Time PC Kit for Novel Coronavirus 2019-nCoV can detect all types of mutations, which included but not limited to Alpha(B.1.1.7+Q), Beta(B.1.351, B.1.351.2, B.1.351.3), Gamma(P.I, P.I.I, P.1.2, P.1.4, P.1.6, P.I.7), Delta(B.1.617.2+AY.x), Eta(B.1.525), Lota(B.1.526), Kappa(B.1.617.1), Lambda(C.37), Zeta(P.2), Omicron(B.1.1.529,BA.1,BA.2,BA.3),etc
Referring to the newly emerged variants of the novel coronavirus, our silicon analysis have proved the target region alignment of our Real-Time PC Kit for Novel Coronavirus 2019-nCoV (Cat#R030-02.R033-01.R033-02.R033-03.R033-04.R034-01.R034-02.R034-03,R034-04), which showed that Uni-medica kits can detect all the above variants without false-negative results off-target situations.
As usual, we will continue to track and update the latest viral information including mutations and substitutions for SARS-CoV-2 to maintain the specificity and sensitivity of our products.
Thanks for your trust and support.
Total solution for Novel Coronavirus detection include:
Novel coronavirus nucleic acid detection kit
Novel coronavirus nucleic acid detection kit
Novel coronavirus nucleic acid rapid test kit
Novel coronavirus mutant nucleic acid detection kit
Novel coronavirus/Flu A/ Flu B nucleic acid detection kit
Respiratory 15/23 pathogen nucleic acid detection kit
Novel coronavirus whole-genome sequencing kit
SARS-COV-2 Antigen Test Kit(Colloida Gold)
SARS-COV-2 IgM/IgG Test Kit(Colloida Gold)
SARS-COV-2 Neutralizing Antibody Rapid Test Kit(Colloida Gold)
We also provide freeze-dried detection kit, making convenient for its transportation under room temperature.
Our high-sensitivity novel coronavirus NA detection kits have been certified by China NMPA and won bidding several times in provincial centralized procurement at home, also CE marked, registered in Philippine,Inida, Peru, Dubai and Iran, and exporter to many countries, supporting globally prevention of pathogen microorganism diseases.